Abstract
Background
Fostamatinib, a spleen tyrosine kinase inhibitor, has been approved for the treatment of chronic immune thrombocytopenia (ITP) in the US, Canada, and Europe. We conducted a phase 3, multi-center study to evaluate the efficacy and safety of fostamatinib in Japanese patients with ITP. The study was composed of 3 treatment periods and 1 non-dosing period: a placebo-controlled, double-blind study of fostamatinib for 24 weeks, an open-label study of fostamatinib for 28 weeks, a non-dosing period of up to 4 weeks, and the voluntary open-label study of fostamatinib until official approval. Here we report the results of the 24-week, placebo-controlled, double-blind, parallel-group study.
Methods
The study enrolled Japanese patients, ≥20 years old, with ITP for ≥6 months, and who had failed or not tolerated ≥1 prior ITP treatment. The mean of 3 screening and baseline platelet counts had to be <30,000/μL, with a platelet count at each visit < 35,000/μL. Patients were randomised 2:1 to fostamatinib or placebo for 24 weeks, stratified by baseline platelet count (< or ≥ 15,000/μL). Dosing was at 100 mg bid for 4 weeks and then 150 mg bid if needed and tolerated. One concomitant treatment was allowed (corticosteroids, azathioprine or danazol).
Results
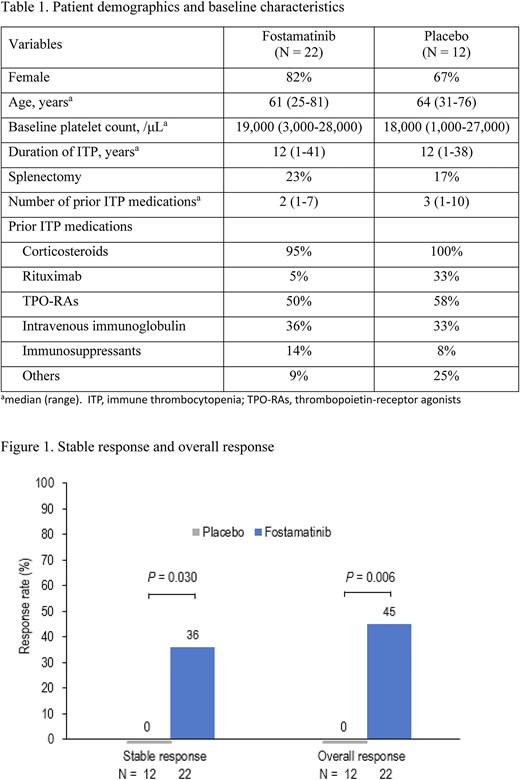
The study included 34 patients, fostamatinib (n=22) or placebo (n=12). See Table 1 for baseline characteristics. Stable responses (platelets ≥50,000/μL at ≥4 of 6 visit, weeks 14-24) were observed in 8 (36%) patients on fostamatinib and 0 patients on placebo (P=0.030) (Figure 1). Overall responses (platelets ≥50,000/μL at ≥1 visit, weeks 2-12) were seen in 10 (45%) patients on fostamatinib and 0 patients on placebo (P=0.006). The median platelet counts of stable responders were higher than those of non-responders and patients on placebo throughout weeks 2 to 24. The median platelet counts of responders on fostamatinib increased ≥50,000/μL from the early stage of fostamatinib administration and stayed at around 100,000/μL from weeks 14 to 24, whereas non-responders on fostamatinib and patients on placebo remained below 30,000/μL most of the time. No platelet overshoot (> 400,000/μL) was observed. Rescue medication was required less often with fostamatinib, and fewer bleeding symptoms occurred with fostamatinib than with placebo. Adverse events in ≥10% of patients on fostamatinib were diarrhoea, hypertension, and decreased neutrophil count. Most adverse events were mild or moderate and manageable. There was no difference in the incidence of infection between the fostamatinib and placebo group; no patients developed a severe infection. No thromboembolic events were reported. The safety profile was consistent with prior studies of fostamatinib.
Conclusions
This study demonstrated the efficacy and safety of fostamatinib in Japanese patients with ITP, including those who had failed or were intolerant to other approved ITP therapies. No new safety signals were identified in Japanese patients with ITP.
Disclosures
Ito:Ono Pharmaceutical Co., Ltd.: Research Funding; Fujimoto Pharmaceutical Corporation: Research Funding; Novartis Pharma K.K.: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding; Celgene K.K.: Honoraria; Takeda Pharmaceutical Company, Limited: Honoraria; AbbVie GK: Honoraria, Research Funding. Hatta:Kyowa Kirin Co: Honoraria, Speakers Bureau; Bristol-Myers Squibb: Honoraria; Novartis Pharma: Honoraria. Fujimaki:Bristol-Myers Squibb: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Otsuka Pharmaceuticals: Honoraria; AbbVie GK: Honoraria; Janssen Pharmaceutical KK: Honoraria; Nippon Shinyaku: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Meiji Seika Pharma Co., Ltd.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; CSL Behring K.K.: Honoraria. Shichiri:Kissei Pharmaceutical Co., Ltd.: Current Employment. Saotome:Kissei Pharmaceutical Co., Ltd.: Current Employment. Masuda:Rigel Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Tomiyama:Kyowa Kirin Co., Ltd.: Honoraria, Speakers Bureau; MEDICAL & BIOLOGICAL LABORATORIES CO., LTD.: Consultancy; Sysmex Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SobiTM Japan: Consultancy; Novartis Pharma K.K.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kissei Pharmaceutical Co., Ltd.: Consultancy; Chugai Pharmaceutical Co., Ltd.: Honoraria; UCB Japan Co. Ltd.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal